Hydration (solvation)
The keyword for ERmod, the application featured in this issue, is "hydration (solvation)." This is a phenomenon that has tremendous significance.
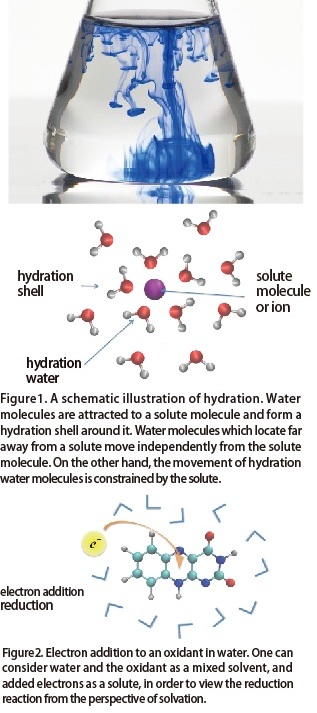 What is hydration?
What is hydration?
When a substance dissolves in water, the dissolved substance (a solute) is stabilized by being surrounded with water molecules. This process is called "hydration." When we say that a certain substance is exceptionally soluble, we mean that it is hydrated well. When hydration occurs, the properties of the solute molecules change. In addition, the reactive properties and stability of the water molecules around the solute also change as compared to when the solute is not present (and as compared to water molecules located far away from the solute). The phenomenon of hydration plays a crucial role in a variety of situations such as the manifestation of protein functions.
Solute molecules do not dissolve only in water. In general, when a substance (solute) dissolves in other molecular assembly systems (solvent), the interaction between the solute and the solvent produces changes in the properties of both solute and solvent. This is known as solvation, and in particular when the solvent is water, it is called hydration.
Expanding the concept of solvation
The concept of solvation can be expanded to consider phenomena other than so-called "dissolution." For example, heterogeneous miscible systems that are made up of water + biological membranes can be seen as solvents, and proteins can be seen as solutes, in order to consider the bonding of proteinsto embranes from the standpoint of solvation.
Additionally, in reduction reactions, the electrons that are added can be seen as solutes, and the substances that receive the electrons and the surrounding medium can be collectively seen as a solvent, in order to enable the concept of solvation to be introduced.
In this way, the concept of "solvation" is expected to enable a unified understanding of various phenomena relating to dissolution, bonding, absorption and so on.