|
I would like to begin by discussing artificial photosynthesis, a research area in which I am currently engaged. Artificial photosynthesis is a system having a mechanism, by which sunlight and water as electron source are used to reduce materials to produce high-energy ones such as hydrogen and carbohydrates. Unlike solar cells, the artificial photosynthesis could extract desired amount of energy when needed. The reduction processes with water as the electron source would not produce any pollutants at all. From the viewpoint of materials circulation as well, this method is thought to provide an ideal next-generation energy system.
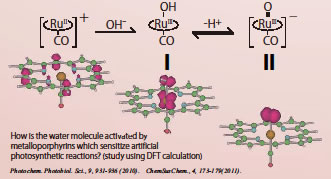
Nevertheless, a challenge to even partly mimic the ingenious mechanisms of the natural system is not so easy. In natural photosynthesis, multiple photons are harvested in antenna system, and the photons are efficiently utilized to activate the oxygen-evolving complex into a highly oxidized form that enables the oxygen evolution. The highly oxidized state is amazingly maintained during the reaction of four-electron oxidation of water. In our research, we are focusing our attention on one-photon excitation of the artificial system that induces two-electron oxidation reactions. In recent years, there has been a major breakthrough, and the actual realization of artificial photosynthesis with water is now almost on the corner. We are now working on the elucidation of the reaction mechanism in molecular level. How the water molecule is activated in the one-photon/two-electron oxidation reaction should be the central subject to be clarified. Theoretical calculation using the density-functional approach has been a very powerful tool in the study.
Japan is one of the leading countries in the world in the area of theoretical chemistry. Nevertheless, if theoreticians alone try to approach any scientific subjects only from a theoretical viewpoint, the results may tend to lack a “reality” of state of the arts in experimental science. In order to not merely develop techniques of calculation but to verify and make them into universal tools, I think researchers, who can step back a bit from the techniques themselves and take a so-called “interpreter’s” perspective, would be indispensable for the key-points. The Division Researchers at CMSI who pursue research in multiple research groups on the cutting edge of the corresponding field would be expected not to merely share information and to have communication among the groups and other fields, but would develop into this new type of researcher. In the future, what if ̶ for example ̶ the framework would expand, so researchers would not only remain at CMSI but also take up residence at an experimental chemist’s laboratory for three months or so in response to a proposal? Actually, theoretical calculations of electronic states on a very high level are already being carried out at experimental chemistry laboratories. The greater progress would be expected in experimental analysis than is now being done, if the support of theoretical chemists could be obtained at the initial stages of calculation. Conversely, the opportunity to learn the essence of experimental chemistry through such a “give-and-take” manner with experimental chemists would be a great stimulus to theoretical chemists as well. Furthermore, it would undoubtedly open up new fields of study. The mechanisms at each level of time and space axis on an actual material are now going to be elucidated, and then those mechanisms would be correlated with each other and be generalized to lead to prediction and design of new materials. Experimental chemists, too, have high hopes for the future advances of computational materials science. (August 11, 2011 at the Advanced Institute
for Computational Science)
|