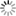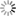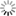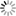Microscopic mechanism of dissociation process of methane hydrate
Takuma Yagasaki
Graduate School of Natural Science and
Technology, Okayama University

Methane hydrate is a solid comprising hydrogen-bonded water cages, which trap methane molecules. Nowadays, many efforts have been made to obtain natural gas from methane hydrates in marine sediments by dissociating them undersea. The dissociation of undersea methane hydrate is also important in atmospheric sciences because it is related to global warming. The mechanism of the hydrate dissociation is still unclear at the molecular level, although it is an important process in many fields of science. I'm now trying to clarify this issue.
One of the most exciting things in research is observing unexpected phenomena. I got this fortune in this study. In general, the melting temperature of a solid is determined by the bond strength between particles, and does not depend on the surrounding environment. However, our molecular dynamics simulations revealed that the melting temperature of methane hydrate in liquid water is significantly higher than that in methane gas at the same pressure.
When the hydrate dissociation occurs in liquid water, released methane molecules immediately become solvated in water. However, this process is highly suppressed because of the very low solubility of methane. This is the reason for the higher melting temperature in water. This mechanism is unique to gas hydrates and cannot occur in usual one-component solids. It was expected from the mechanism that bubble formation causes an increase in the dissociation rate. We have confirmed this in the following calculations.
Our mechanism suggests inhomogeneous melting of methane hydrate due to bubbles. However, it is difficult to examine this phenomenon by small-system simulations. Effects of heat transport, gradient of methane concentration, and translation of bubbles are important for the hydrate dissociation, but analyses of them also require large-system simulations. We will address these issues by using the K computer.
 |
|---|
| A snapshot in the course of the methane hydrate dissociation in water. Released methane molecules form a bubble. This bubble enhances further dissociation. |