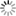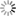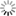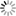Fundamental study for development of high-performance Li-ion battery
Interviewee:
|
Interviewer:
|
| Researcher, Nanosystem Research Institute, National Institute of Advanced Industrial Science and Technology (AIST) |
CMSI Priority Area Researcher |
The challenge of finding our way to sustainable energies is putting an increasing demand on computational studies. In our group, we are using the K computer with the objective to shed light on a key mechanism that takes place in lithium-ion batteries.
The emergence of a sustainable, non CO₂-emitting, energy economy depends heavily on the development of autonomous power systems for application in smart-grid energy storage and transportation. In this regard, Li-ion batteries are a leading technology whose performance‒measured in terms of energy density, power density, recharging time, durability, cost, and safety‒depends critically on the structure and composition of the electrodes and electrolyte. In particular, the properties of the solid-electrolyte interface (SEI)‒the specific phase that results from the chemical decomposition of the organic solvent on the surface of the anode‒are known to be very sensitive to the insertion of small quantities of chemical additives into the electrolyte. Because, in addition, the SEI is known to simultaneously control the electrode resistance against corrosion and the Li-ion insertion-extraction rate, a detailed knowledge of the SEI structure is essential in trying to reduce the degradation of the battery discharge capacity upon successive charge-discharge cycles.
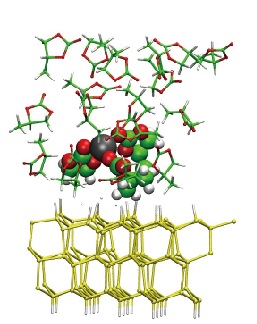
In practice however, the SEI is hard to characterize experimentally, implying a strong interest in directly simulating the SEI formation mechanism in the computer. Recently, we have used the first-principles code Open-MX+ESM on the K computer to run 500-atom simulations in which the anode is represented by a slab made of silicon‒that has higher Li insertion capacity than carbon‒and the electrolyte is composed of one molecule of LiPF₆ salt dissolved in propylene carbonate. When putting the system under bias, we have been able to directly observe the progressive migration, desolvation, and adsorption of the Li ion on the surface of the electrode. Following these promising results, we are now conducting simulations containing up to 4000 atoms, which will allow us to assess the critical effect of ppm concentrations of chemical additives and/or traces of water on the Li-ion insertion process and SEI formation mechanism.
|
|
| Snapshot from MD simulation of the electrode/electrolyte interface of a lithium-ion battery: desolvation of the lithium ion (in black) from propylene carbonate in the vicinity of the electrode. |